NIST researchers use cellphone compass to measure glucose
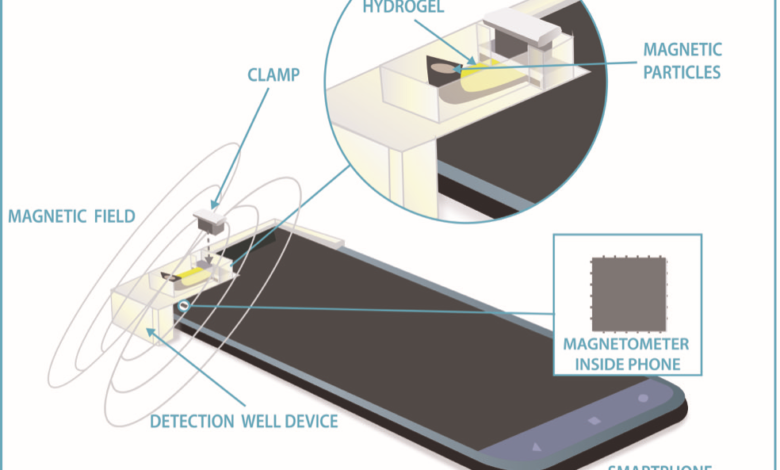
- Smartphones can now measure glucose, a key marker for diabetes, as well as other molecules and biomarkers, thanks to their built-in magnetic compasses.
- NIST researchers added magnetic particles to a porous material that reacts to the presence of these molecules and causes the material to expand or contract.
- Using these magnetized materials in conjunction with cellphone compasses could potentially enable individuals to diagnose disease and detect environmental toxins.
Nearly every modern cellphone has a built-in compass, or magnetometer, that detects the direction of Earth’s magnetic field, providing critical information for navigation. Now a team of researchers at the National Institute of Standards and Technology (NIST) has developed a technique that uses an ordinary cellphone magnetometer for an entirely different purpose — to measure the concentration of glucose, a marker for diabetes, to high accuracy.
The same technique, which uses the magnetometer in conjunction with magnetic materials designed to change their shape in response to biological or environmental cues, could be used to rapidly and cheaply measure a host of other biomedical properties for monitoring or diagnosing human disease. The method also has the potential to detect environmental toxins, said NIST scientist Gary Zabow.
In their proof-of-concept study, Zabow and fellow NIST researcher Mark Ferris clamped to a cellphone a tiny well containing the solution to be tested and a strip of hydrogel — a porous material that swells when immersed in water. The researchers embedded tiny magnetic particles within the hydrogel, which they had engineered to react either to the presence of glucose or to pH levels (a measure of acidity) by expanding or contracting. Changing pH levels can be associated with a variety of biological disorders.

Credit:
K. Dill/NIST
As the hydrogels enlarged or shrunk, they moved the magnetic particles closer to or farther from the cellphone’s magnetometer, which detected the corresponding changes in the strength of the magnetic field. Employing this strategy, the researchers measured glucose concentrations as small as a few millionths of a mole (the scientific unit for a certain number of atoms or molecules in a substance). Although such high sensitivity is not required for at-home monitoring of glucose levels using a drop of blood, it might in the future enable routine testing for glucose in saliva, which contains a much smaller concentration of the sugar.
The researchers reported their findings in the March 30, 2024 edition of Nature Communications.
Engineered, or “smart,” hydrogels like the ones the NIST team employed are inexpensive and relatively easy to fabricate, Ferris said, and can be tailored to react to a host of different compounds that medical researchers may want to measure. In their experiments, he and Zabow stacked single layers of two different hydrogels, each of which contracted and expanded at different rates in response to pH or glucose. These bilayers amplified the motion of the hydrogels, making it easier for the magnetometer to track changes in magnetic field strength.
Because the technique does not require any electronics or power source beyond that of the cellphone nor call for any special processing of the sample, it offers an inexpensive way to conduct testing — even in locations with relatively few resources.
Future efforts to improve the accuracy of such measurements using cellphone magnetometers might allow detection of DNA strands, specific proteins and histamines — compounds involved in the body’s immune response — at concentrations as low as a few tens of nanomoles (billionths of a mole).
That improvement could have substantial benefit. For instance, measuring histamines, which are typically detected in urine at concentrations ranging from about 45 to 190 nanomoles, would ordinarily require a 24-hour urine collection and a sophisticated laboratory analysis.
“An at-home test using a cellphone magnetometer sensitive to nanomolar concentrations would allow measurements to be done with much less hassle,” said Ferris. More generally, enhanced sensitivity would be essential when only a small amount of a substance is available for testing in extremely dilute quantities, Zabow added.
Similarly, the team’s study suggests that a cellphone magnetometer can measure pH levels with the same sensitivity as a thousand-dollar benchtop meter but at a fraction of the cost. A home-brewer or a baker could use the magnetometer to quickly test the pH of various liquids to perfect their craft, and an environmental scientist could measure the pH of ground water samples on-site with higher accuracy than a litmus test strip could provide.
In order to make the cellphone measurements a commercial success, engineers will need to develop a method to mass produce the hydrogel test strips and ensure that they have a long shelf life, Zabow said. Ideally, he added, the hydrogel strips should be designed to react more quickly to environmental cues in order to speed up measurements.
Paper: Mark Ferris and Gary Zabow. Quantitative, High Sensitivity Measurement of Liquid Analytes using a Smartphone Compass. Nature Communications. Published online March 30, 2024. DOI: https://doi.org/10.1038/s41467-024-47073-2


