Xbox Could Be Preparing To Add Two Big EA Releases To Game Pass

We’re expecting NHL 25 soon as well

Anyone for another roundup of Xbox Game Pass rumours? The latest comes courtesy of EA, as it appears that both Dragon Age: The Veilguard and EA Sports FC 25 are being prepared for Xbox Game Pass at some point in the future.
Dragon Age: The Veilguard is the most surprising one here, as the game’s only been out for just under six months, but as spotted by the likes of Knobel and Xbox Era, an apparently new Xbox Store listing has appeared on PC with the words “PC Game Pass” alongside it. Here’s a closer look:
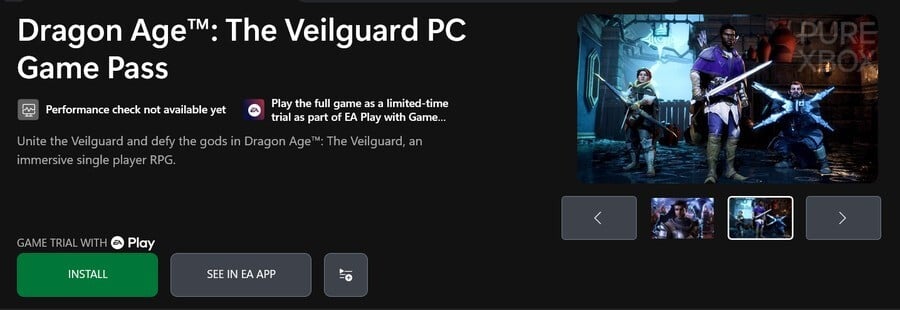
The bit we’re unsure about here is that the “PC Game Pass” mention might just refer to the free EA Play trial that’s included with the game, but the fact that it’s supposedly a new listing suggests something bigger might be in the works.
EA Sports FC 25 makes a lot of sense, of course, as those games are always added to EA Play and Xbox Game Pass Ultimate somewhere around the May-June time period. We expect that to be the case again this year.
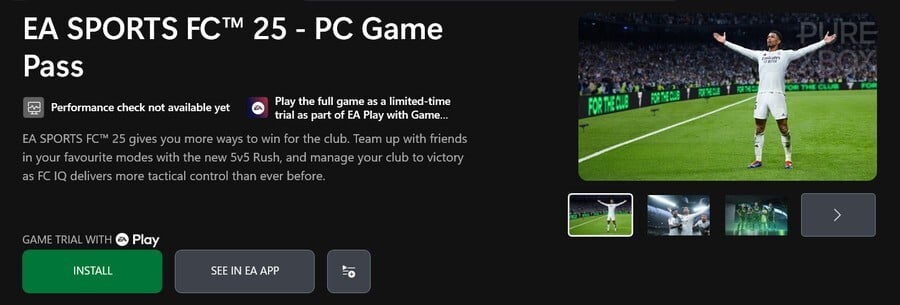
Don’t take either of these as confirmation for Xbox Game Pass yet, but we’d say EA Sports FC 25 is very likely for the next few months, and we’re hopeful but not entirely confident about Dragon Age: The Veilguard. Don’t forget NHL 25 will probably be added for console players alone at some point in April or May as well!
Would you want to play either of these on Xbox Game Pass? Tell us down below.
![]()
Fraser is the News Editor at Pure Xbox, where he spends his time reporting on the biggest stories in the world of Xbox and beyond.



