Clinical Challenges: Oral Immunotherapy for Food Allergies
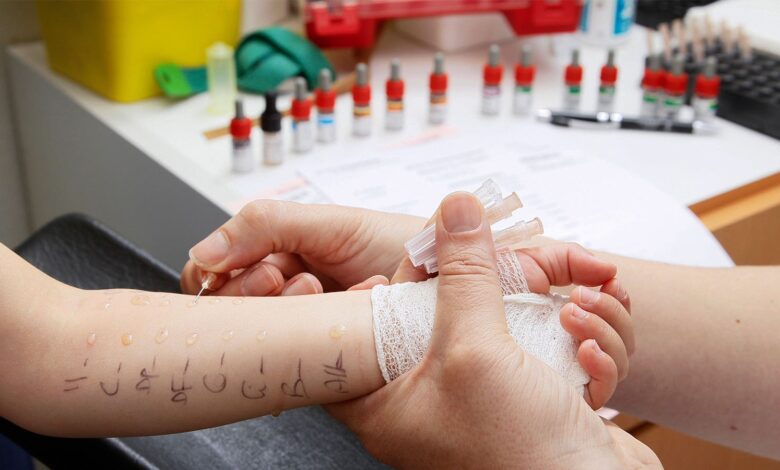
— Long waitlists, low reimbursement among frustrations reported in real world
by
Elizabeth Short, Staff Writer, MedPage Today
January 10, 2024
Hailed for opening a new chapter in food allergy management, oral immunotherapy (OIT) is nevertheless going through growing pains in accessibility and acceptance in clinical practice.
In 2020, the FDA approved peanut allergen powder (Palforzia), an OIT developed to combat allergic reactions to peanuts — a departure from traditional allergen avoidance and anaphylaxis treatment. The peanut powder, indicated for children ages 4 to 17 years, consists of initial and supplemental doses alongside a peanut avoidance diet and is intended to help mitigate the effects of allergen exposure.
“We are in the middle of a tectonic shift in the management and treatment of food allergy,” said Warner Carr, MD, of the Children’s Hospital of Orange County in California.
Similar products are in development for other nut and food allergies, including ADP101 (manufactured by Alladapt Immunotherapeutics), a multi-food OIT that FDA granted fast track designation in November 2023 to expedite its development as a treatment for children ages 4 and up with one or more food allergies. The product — which contains peanut, almond, cashew, hazelnut, pecan, pistachio, walnut, sesame, soy, wheat, cow’s milk, chicken egg, codfish, salmon, and shrimp allergen proteins — would be administered to patients in controlled doses consistent with current OIT practices.
Patients and their families, meanwhile, have expressed a range of expectations and fears for the relatively new field of OIT.
For example, in an April 2023 focus group, parents of children with peanut allergies reported being eager and “excited” to learn about OIT, often describing themselves as being in “research mode” at the time their child was diagnosed. Among the top caregiver concerns were whether or not their child would be capable of self-reporting their reaction symptoms and whether or not they would go along with prescribed treatment.
Many caregivers also reported feeling “frustrated” with comparing conflicting items of research, as well as dealing with long wait times to see specialists.
Carr told MedPage Today that he is familiar with the struggle to see patients in a timely manner for them to start OIT.
“There is a huge unmet burden of disease. I have a very, very, very busy food allergy center. I have over 500 people on a waiting list,” he said. “If I saw you today, it could take anywhere from 8 to 12 months before you can actually start with treatments. There is a huge burden of disease and there just isn’t enough food allergy experts out there doing this treatment.”
Additionally, costs and reimbursement can be an additional hurdle for providers, even if patients have insurance. “The cost of providing this service is very expensive. And between staff and storage and space, it is a huge barrier,” said Carr.
“The vast majority of our payments come from insurance companies … There is no insurance code for doing oral immunotherapy, we use visit codes and we use challenge codes,” he noted. “The reimbursement for doing oral immunotherapy is very, very low, and that is a major barrier for doctors that own their business, or for other businesses that employ doctors that can do this treatment.”
Besides the high cost, treatment with the approved peanut allergen product requires a dosing schedule involving many office visits and an indefinite home-dosing period.
These time and financial commitments make ensuring that people really have a food allergy before starting them on OIT all the more important, said Kristin Sokol, MD, MPH, of Schreiber Allergy Center in Rockville, Maryland.
“We don’t want to commit them to a full year plus more of oral immunotherapy, a medical protocol, if they’re truly not allergic,” she said. “If it looks like there’s a chance that they might pass a challenge, but we’re not sure, of course we’re going to do a food challenge.”
Sokol described oral food challenges as the “gold standard” of allergy tests. Patients who don’t pass the food challenge may be candidates for OIT, perhaps starting at a somewhat higher dose than for people who did not undergo the food challenge, she told MedPage Today.
In practice, fears around food challenges do lead patients to skip this step before asking for OIT.
Monica Kraft, MD, of the Ohio State University Wexner Medical Center in Columbus, explained to MedPage Today that communication is key to making parents and patients feel more comfortable with oral food challenges.
“I have actually found in the majority of cases, patients are pretty amenable to doing the food challenge once we discuss what it involves and how we try to do it in a safe and controlled way,” she said, emphasizing that “they usually feel a little bit more reassured” after being informed that the allergy clinic where they receive treatment under close monitoring has medications available should an allergic reaction occur.
“I also try to be pretty upfront with why we’re discussing the food challenge,” Kraft added. “Often these are people who have very low-positive allergy test results by skin or blood, or they’re completely negative and they were told in the past they were allergic to the food, so there’s some lack of clarity.”
-
![author['full_name']](https://clf1.medpagetoday.com/media/images/author/EShort_188.jpg)
Elizabeth Short is a staff writer for MedPage Today. She often covers pulmonology and allergy & immunology. Follow
Disclosures
Sokol reported relationships with Sanofi and Genentech.
Carr and Kraft had no disclosures.



