Comorbidities in Midlife Tied to Cancer Risk
—
Strong association was observed for risk of multiple individual cancer types
by
Mike Bassett, Staff Writer, MedPage Today
April 7, 2025
- In a study of people without a history of cancer, comorbidities in midlife were associated with an overall risk of cancer.
- There was a stronger association between comorbidities and risk of multiple individual cancer types.
- The findings support the incorporation of formal comorbidity screening and/or risk assessment as a routine aspect of cancer screening visits.
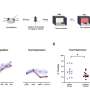
Comorbidities in midlife were associated with a modestly higher risk of cancer overall and more strongly associated with a risk for multiple individual cancer types, according to a secondary analysis of the prospective Prostate, Lung, Colorectal, and Ovarian (PLCO) screening trial.
Among nearly 130,000 participants without a history of cancer, the risk of any incident cancer was significantly higher for adults with a history of respiratory (HR 1.07, 95% CI 1.02-1.12) and cardiovascular conditions (HR 1.02, 95% CI 1.00-1.05), reported Lee W. Jones, PhD, of Memorial Sloan Kettering Cancer Center in New York City, and colleagues.
None of the other comorbidity classifications (gastrointestinal, liver, and metabolic) had a significant association with the risk of cancer overall. However, all of the comorbidity classifications were associated with the risk of multiple individual cancer types, with the direction of that risk differing across types, they noted in JAMA Network Open.
For example, the strongest association was between a history of liver conditions and risk of liver cancer (HR 5.57, 95% CI 4.03-7.71), while liver conditions were associated with a significantly reduced risk of endometrial cancer (HR 0.52, 95% CI 0.31-0.87).
Meanwhile, metabolic conditions (obesity and type 2 diabetes) were associated with an increased risk of liver, endometrial, kidney, biliary, thyroid, rectal, colon, pancreas, and hematopoietic cancers (with HRs ranging from 1.14-2.04), and a decreased risk of melanoma, lung, head and neck, and prostate cancers (with HRs ranging from 0.75-0.91).
“The strong positive associations together with the high global prevalence of obesity and type 2 diabetes further underscores the public health importance of efforts to curtail these conditions,” Jones and colleagues observed.
Additionally, cardiovascular conditions were associated with an increased risk of four cancers (prostate, biliary, upper gastrointestinal, and kidney; HRs ranging from 1.07-1.47) and a decreased risk of breast cancer (HR 0.93). Gastrointestinal conditions were also associated with an increased risk of four cancers (thyroid, breast, kidney, and ovarian; HRs ranging from 1.25-1.50) and a decreased risk of prostate cancer (HR 0.60), while respiratory conditions were associated with an increased risk of lung (HR 1.80) and pancreatic (HR 1.33) cancers, and a reduced risk of prostate cancer (HR 0.70).
“From a clinical perspective, our findings support the incorporation of formal comorbidity screening and/or risk assessment as a routine aspect of cancer screening visits and could be more systematically incorporated into cancer risk calculators,” Jones and team wrote.
Regarding cancer mortality, respiratory conditions were positively associated with a higher hazard of cancer death (HR 1.19, 95% CI 1.11-1.28), as were cardiovascular (HR 1.08, 95% CI 1.04-1.13) and metabolic (HR 1.09, 95% CI 1.05-1.14) conditions.
For individual cancer types, metabolic conditions were associated with a higher hazard of cancer-specific mortality following a diagnosis of endometrial cancer, upper gastrointestinal cancer, hematopoietic cancer, and prostate cancer, while cardiovascular conditions were associated with a higher hazard of death following a diagnosis of hematopoietic cancer and lung cancer.
The authors said that the higher hazard of cancer death among individuals with comorbidities could be due to suboptimal dosing of planned therapy due to tolerability concerns, but also suggested that “the potential importance of comorbidities on cancer mortality also supports … individualized primary prevention among individuals in which an early lesion is detected.”
In a commentary accompanying the study, Siran M. Koroukian, PhD, of Case Western Reserve University in Cleveland, and colleagues argued that there is a need to “further improve” the methods of accounting for co-existing conditions.
“The complexity created by the co-occurrence of these conditions should be viewed in the context of multimorbidity, whereby each condition, not just the index condition, is given attention relative to disease burden and risk assessment, and in care management decisions,” they wrote.
They added that researchers need to adopt a granular approach in characterizing multimorbidity with greater specificity, and that such an approach “will be critical to develop personalized cancer prevention strategies, and subsequently personalized cancer treatment.”
The PLCO screening trial included adults ages 55 to 74 years without a history of cancer who were enrolled between 1993 and 2001. Among the 128,999 participants included in this analysis, median age was 62, 49.7% were men, 88.4% were white, 5.2% were Black, and 4.2% were Asian or Pacific Islander. Median follow-up was 20 years.
For purposes of the study, comorbidities were classified into five specific combinations — cardiovascular conditions (coronary heart disease or heart attack, stroke, and hypertension), gastrointestinal conditions (ulcerative colitis, Crohn’s disease, Gardner syndrome, familial adenomatous polyposis, diverticulitis or diverticulosis, and gallbladder stones or inflammation), respiratory conditions (chronic bronchitis or emphysema), liver conditions (hepatitis or cirrhosis), and metabolic conditions (obesity and type 2 diabetes).
-
![author['full_name']](https://assets.medpagetoday.net/media/images/author/MikeBassett_188.jpg)
Mike Bassett is a staff writer focusing on oncology and hematology. He is based in Massachusetts.
Disclosures
Researchers were supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant, the UCLA Cancer Center Support Grant, and the American Association for Cancer Research Project Genomics Evidence Neoplasia Information Exchange Biopharma Collaborative.
Jones reported owning stock in Illumisonics and Pacylex. A co-author reported being on safety boards for BioSymetrics, Intersect Diagnostics, and Sage Bionetworks.
Koroukian was supported by several government grants, as well as a grant from the American Cancer Society, and by contracts from the Cleveland Clinic Foundation (including a subcontract from Celgene). A co-author also reported receiving government grants.
Primary Source
JAMA Network Open
Source Reference: Lavery JA, et al “Comorbidity in midlife and cancer outcomes” JAMA Netw Open 2025; DOI: 10.1001/jamanetworkopen.2025.3469.
Secondary Source
JAMA Network Open
Source Reference: Koroukian SM, et al “Moving closer to personalized cancer prevention strategies by assessing comorbidity and multimorbidity” JAMA Netw Open 2025; DOI: 10.1001/jamanetworkopen.2025.3476.