Tweaking non-neural brain cells can cause memories to fade
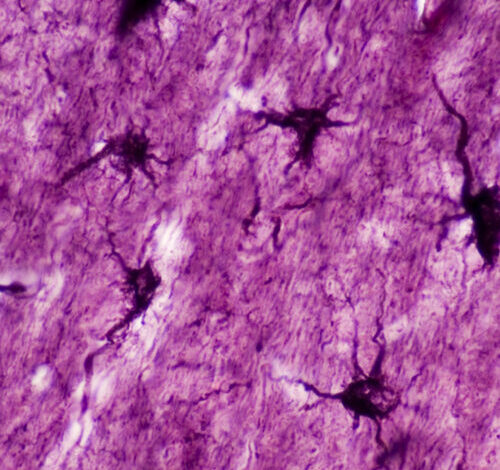
“If we go back to the early 1900s, this is when the idea was first proposed that memories are physically stored in some location within the brain,” says Michael R. Williamson, a researcher at the Baylor College of Medicine in Houston. For a long time, neuroscientists thought that the storage of memory in the brain was the job of engrams, ensembles of neurons that activate during a learning event. But it turned out this wasn’t the whole picture.
Williamson’s research investigated the role astrocytes, non-neuron brain cells, play in the read-and-write operations that go on in our heads. “Over the last 20 years the role of astrocytes has been understood better. We’ve learned that they can activate neurons. The addition we have made to that is showing that there are subsets of astrocytes that are active and involved in storing specific memories,” Williamson says in describing a new study his lab has published.
One consequence of this finding: Astrocytes could be artificially manipulated to suppress or enhance a specific memory, leaving all other memories intact.
Marking star cells
Astrocytes, otherwise known as star cells due to their shape, play various roles in the brain, and many are focused on the health and activity of their neighboring neurons. Williamson’s team started by developing techniques that enabled them to mark chosen ensembles of astrocytes to see when they activate genes (including one named c-Fos) that help neurons reconfigure their connections and are deemed crucial for memory formation. This was based on the idea that the same pathway would be active in neurons and astrocytes.
“In simple terms, we use genetic tools that allow us to inject mice with a drug that artificially makes astrocytes express some other gene or protein of interest when they become active,” says Wookbong Kwon, a biotechnologist at Baylor College and co-author of the study.
Those proteins of interest were mainly fluorescent proteins that make cells fluoresce bright red. This way, the team could spot the astrocytes in mouse brains that became active during learning scenarios. Once the tagging system was in place, Williamson and his colleagues gave their mice a little scare.
“It’s called fear conditioning, and it’s a really simple idea. You take a mouse, put it into a new box, one it’s never seen before. While the mouse explores this new box, we just apply a series of electrical shocks through the floor,” Williamson explains. A mouse treated this way remembers this as an unpleasant experience and associates it with contextual cues like the box’s appearance, the smells and sounds present, and so on.
The tagging system lit up all astrocytes that expressed the c-Fos gene in response to fear conditioning. Williamson’s team inferred that this is where the memory is stored in the mouse’s brain. Knowing that, they could move on to the next question, which was if and how astrocytes and engram neurons interacted during this process.
Modulating engram neurons
“Astrocytes are really bushy,” Williamson says. They have a complex morphology with lots and lots of micro or nanoscale processes that infiltrate the area surrounding them. A single astrocyte can contact roughly 100,000 synapses, and not all of them will be involved in learning events. So the team looked for correlations between astrocytes activated during memory formation and the neurons that were tagged at the same time.
“When we did that, we saw that engram neurons tended to be contacting the astrocytes that are active during the formation of the same memory,” Williamson says. To see how astrocytes’ activity affects neurons, the team artificially stimulated the astrocytes by microinjecting them with a virus engineered to induce the expression of the c-Fos gene. “It directly increased the activity of engram neurons but did not increase the activity of non-engram neurons in contact with the same astrocyte,” Williamson explains.
This way his team established that at least some astrocytes could preferentially communicate with engram neurons. The researchers also noticed that astrocytes involved in memorizing the fear conditioning event had elevated levels of a protein called NFIA, which is known to regulate memory circuits in the hippocampus.
But probably the most striking discovery came when the researchers tested whether the astrocytes involved in memorizing an event also played a role in recalling it later.
Selectively forgetting
The first test to see if astrocytes were involved in recall was to artificially activate them when the mice were in a box that they were not conditioned to fear. It turned out artificial activation of astrocytes that were active during the formation of a fear memory formed in one box caused the mice to freeze even when they were in a different one.
So, the next question was, if you just killed or otherwise disabled an astrocyte ensemble active during a specific memory formation, would it just delete this memory from the brain? To get that done, the team used their genetic tools to selectively delete the NFIA protein in astrocytes that were active when the mice received their electric shocks. “We found that mice froze a lot less when we put them in the boxes they were conditioned to fear. They could not remember. But other memories were intact,” Kwon claims.
The memory was not completely deleted, though. The mice still froze in the boxes they were supposed to freeze in, but they did it for a much shorter time on average. “It looked like their memory was maybe a bit foggy. They were not sure if they were in the right place,” Williamson says.
After figuring out how to suppress a memory, the team also figured out where the “undo” button was and brought it back to normal.
“When we deleted the NFIA protein in astrocytes, the memory was impaired, but the engram neurons were intact. So, the memory was still somewhere there. The mice just couldn’t access it,” Williamson claims. The team brought the memory back by artificially stimulating the engram neurons using the same technique they employed for activating chosen astrocytes. “That caused the neurons involved in this memory trace to be activated for a few hours. This artificial activity allowed the mice to remember it again,” Williamson says.
The team’s vision is that in the distant future this technique can be used in treatments targeting neurons that are overactive in disorders such as PTSD. “We now have a new cellular target that we can evaluate and potentially develop treatments that target the astrocyte component associated with memory,” Williamson claims. But there’s lot more to learn before anything like that becomes possible. “We don’t yet know what signal is released by an astrocyte that acts on the neuron. Another thing is our study was focused on one brain region, which was the hippocampus, but we know that engrams exist throughout the brain in lots of different regions. The next step is to see if astrocytes play the same role in other brain regions that are also critical for memory,” Williamson says.
Nature, 2024. DOI: 10.1038/s41586-024-08170-w
Jacek Krywko is a freelance science and technology writer who covers space exploration, artificial intelligence research, computer science, and all sorts of engineering wizardry.




